Activation of CO2 by tBuZnOH species: efficient routes to novel nanomaterials based on zinc carbonates†
Abstract
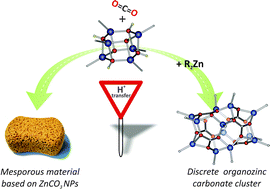
We report on the activation of CO2 by the well-defined alkylzinc hydroxide (tBuZnOH)6 in the absence and presence of tBu2Zn as an external

 Please wait while we load your content...
Please wait while we load your content...