Unconventional preparation of racemic crystals of isopropyl 3,3,3-trifluoro-2-hydroxypropanoate and their unusual crystallographic structure: the ultimate preference for homochiral intermolecular interactions†
Abstract
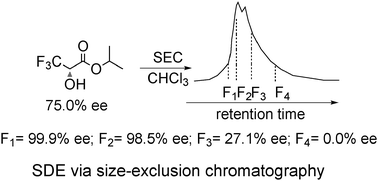
This work reports the first example of the transformation of conglomerate (R)- and (S)-crystals into racemic crystals via

 Please wait while we load your content...
Please wait while we load your content...