Enantioselective construction of vicinal all-carbon quaternary centers via catalytic double asymmetric decarboxylative allylation†‡
Abstract
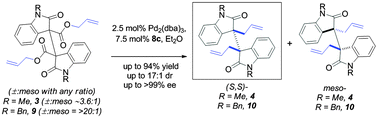
We report a catalytic stereoconvergent construction of vicinal all-carbon quaternary centers via double stereoablative enantioselective alkylation of a mixture having racemic and meso diastereomers of esters to afford exceptional levels of diastereo- (up to 17 : 1) and enantioselectivity (up to >99% ee). The strategy offers an efficient and general approach to dimeric cyclotryptamine alkaloids sharing a labile C3a–C3a′ σ-bond in the hexahydropyrroloindoline core.

 Please wait while we load your content...
Please wait while we load your content...