An unexpected stereoisomerism in enantiomerically pure trisadducts of C60 with an inherently chiral trans-3,trans-3,trans-3 addition pattern†
Abstract
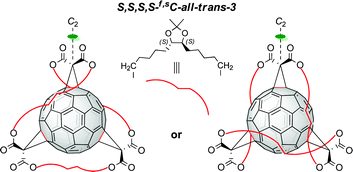
We have synthesized and isolated two C2-symmetric enantiomerically pure trisadducts of C60 with an inherently chiral trans-3,trans-3,trans-3 addition pattern with the aid of an enantiopure cyclo-tris-malonate tether. An unexpected stereoisomerism was observed which was attributed to the spatial arrangement of spacers in the tether.

 Please wait while we load your content...
Please wait while we load your content...