C–H functionalization of tetramethylsilane employing a borylnitrene†
Abstract
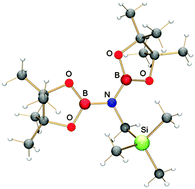
The irradiation of 2-azido-4,4,5,5-tetramethyl-1,3,2-dioxaborol (pinBN3, pin = pinacolato) generates the corresponding borylnitrene that easily inserts into the CH bond of tetramethylsilane. This primary photoproduct, a monoborylated aminoborane, undergoes a subsequent reaction with pinBN3 forming a bisborylated aminoborane.

 Please wait while we load your content...
Please wait while we load your content...