Enantioselective desymmetrization of prochiral 1,3-dinitropropanes via organocatalytic allylic alkylation†
Abstract
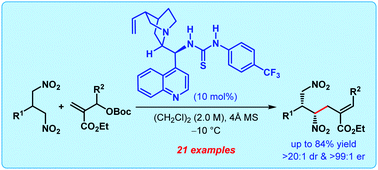
An enantioselective desymmetrization of prochiral 1,3-dinitropropanes has been developed which proceeds via enantiogroup differentiating organocatalytic allylic alkylation. Densely functionalized products with two vicinal stereocenters were obtained generally with good to excellent diastereoselectivity (up to >20 : 1 dr) and superb enantioselectivity (up to >99 : 1 er).

 Please wait while we load your content...
Please wait while we load your content...