Diverse reactions of N-heterocyclic carbenes with an alkynylborane and isolation of a reactive zwitterionic borataallene†
Abstract
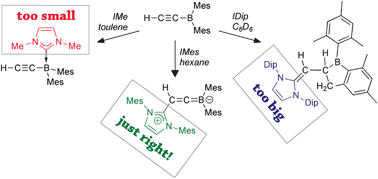
Ethynyldimesitylborane (1) is synthesised via salt elimination and its reactivity towards NHCs is studied. Depending on their size, NHCs attack either at the boron atom or at the β-alkynyl carbon atom. Steric control over the reaction was probed by reactions with N-heterocyclic carbenes yielding a carbene–borane adduct (2), a 1-boraindane (3), and the first structurally characterised borataallene (4).

 Please wait while we load your content...
Please wait while we load your content...