Activation of cell-penetrating peptides by disulfide bridge formation of truncated precursors†
Abstract
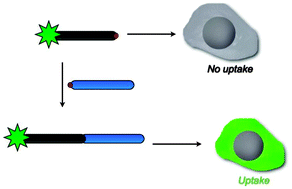
A small library of oligoarginine peptides equipped with terminal cysteines was studied with respect to their cell-penetrating properties. The peptides themselves were inactive but gained the ability to enter cells upon extension of their sequence through disulfide bridge formation.

 Please wait while we load your content...
Please wait while we load your content...