Engineered P450pyr monooxygenase for asymmetric epoxidation of alkenes with unique and high enantioselectivity†
Abstract
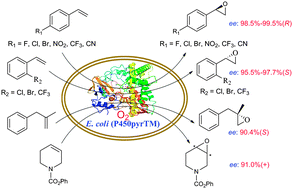
A triple mutant of P450pyr monooxygenase (P450pyrTM) catalysed the epoxidation of several para-substituted styrenes as the first enzyme showing high (R)-enantioselectivity and high conversion, demonstrated a broad substrate range, and showed high enantioselectivity for the epoxidation of an unconjugated 1,1-disubstituted alkene, 2-methyl-3-phenyl-1-propene, and a cyclic alkene, N-phenoxycarbonyl-1,2,5,6-tetrahydropyridine, respectively.

 Please wait while we load your content...
Please wait while we load your content...