Abstract
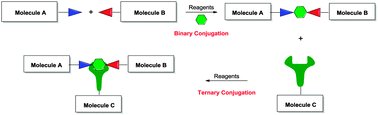
Conjugation is an important reaction that enables coupling of molecules. Many protocols exist for the synthesis of binary conjugates from two different molecules or for the polyvalent display of a single molecule. There aren't many methods for the synthesis of ternary conjugates. However, methods for ternary conjugation are important for understanding the interplay of interactions between three biomolecules (or any three molecules per se). A strategy for ternary bioconjugation using inverse electron demand Diels–Alder reaction with tetrazine is studied. Ternary conjugation was demonstrated by the reaction of a model glyco-peptide binary conjugate with a fluorescent tagged olefin.

 Please wait while we load your content...
Please wait while we load your content...