Chiral silicon Lewis acids having a pentacoordinate stereogenic silicon center: 29Si NMR studies and application to asymmetric Diels–Alder reactions†
Abstract
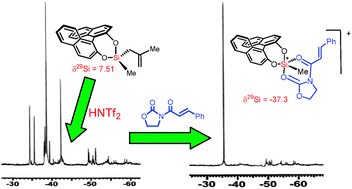
The 29Si NMR studies of chiral pentacoordinate silyl triflimides having a stereogenic center at silicon have revealed that a chiral silicon center is highly configurationally unstable. Such configurational instability has an enormously beneficial effect on the diastereo- and enantioselectivity of the catalytic asymmetric Diels–Alder reaction.

 Please wait while we load your content...
Please wait while we load your content...