An efficient method for the Heck–Catellani reaction of aryl halides†
Abstract
We describe herein a new method that allows selective production of Catellani–Heck isomers from various aryl halides, including ones without ortho-groups. Under previous conditions, unhindered aryl halides were plagued with the formation of simple Heck isomers and multiple arylation and norbornene insertion. The use of a bulky PtBu3 ligand accelerates C–C reductive elimination from the key palladacycle.
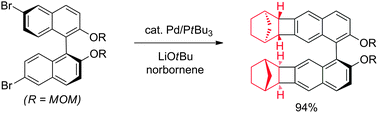

 Please wait while we load your content...
Please wait while we load your content...