Recent efforts to construct the B-ring of bryostatins
Abstract
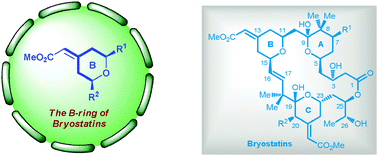
Among macrocyclic natural products, bryostatins have excellent bioactivities and unique structures that make them highly attractive to synthetic chemists. Particularly challenging for the total synthesis of bryostatins is the B-ring, which features a cis-tetrahydropyran containing a geometrically defined exocyclic Z-methyl enoate. Synthetic chemists have recently displayed great prowess in their efforts to construct this ring, and here we summarize the progress towards this goal.

 Please wait while we load your content...
Please wait while we load your content...