Reactions of an isolable dialkylsilylene with aromatic nitriles providing a new type of heterosilole†
Abstract
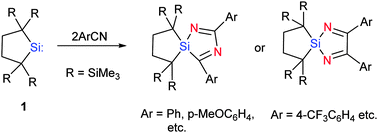
The 1 : 2 reactions of isolable dialkylsilylene 1 with nitriles having electron-donating aromatic substituents gave 1,4-diaza-2-siloles with a hitherto-unknown type of ring system, in contrast to the previous studies showing exclusive formation of the corresponding 1,3-diaza-2-siloles; the reactions of 1 with aromatic nitriles bearing electron-withdrawing substituents afforded the latter ring system. The remarkable diversity of the reactions is explained by invoking the corresponding nitrile silaylides as key intermediates whose polarity switches depending on the substituents of nitriles.

 Please wait while we load your content...
Please wait while we load your content...