Design of supramolecular amino acids to template peptide folding†
Abstract
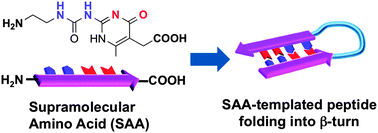
We describe in this manuscript a new design of supramolecular amino acids (SAAs) for directing peptide folding in organic environments. The incorporated supramolecular motif has a strong driving force to dimerize in a sequence- and orientation-specific manner. By introducing such SAAs into the primary sequence of peptides, the specific and directional dimerization of the supramolecular units should facilitate the folding of the peptides. Our approach may provide a general strategy to program secondary structures in organic media.

 Please wait while we load your content...
Please wait while we load your content...