Pro-Gly mediated conformational switch of mycobacteriophage D29 holin transmembrane domain I is lipid concentration driven†
Abstract
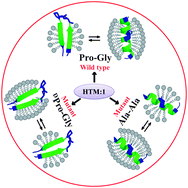
Biophysical and spectroscopic analysis of synthetic transmembrane domain I (TM1) of mycobacteriophage D29 holin shows a lipid concentration dependent conformational switch from an α-helix to a β-sheet structure. The reversibility of this switch, upon change in the lipid-to-peptide ratio, requires a central Pro-Gly segment, and is abolished upon mutation to Ala-Ala or DPro-Gly.

 Please wait while we load your content...
Please wait while we load your content...