Chiral suprastructures of asymmetric oligothiophene-hybrids induced by a single proline†
Abstract
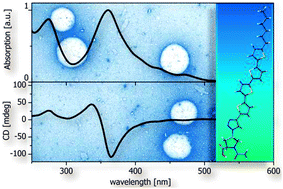
Oligothiophene–proline hybrids were synthesized via click-reaction showing intriguing self-assembly behavior in an aqueous environment by forming chiral superstructures, whose helicity is controlled by the configuration of the amino acid moiety.

 Please wait while we load your content...
Please wait while we load your content...