Synthesis of cyclic peptide hemicryptophanes: enantioselective recognition of a chiral zwitterionic guest†
Abstract
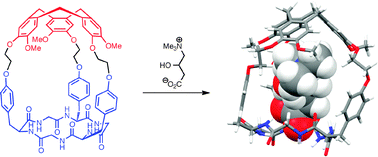
The synthesis of the first members of a new class of cyclic peptide-containing hemicryptophanes is described. Synthesis was achieved through attachment of veratryl groups to the

 Please wait while we load your content...
Please wait while we load your content...