Quantitation of Li2O2 stored in Li–O2 batteries based on its reaction with an oxoammonium salt†
Abstract
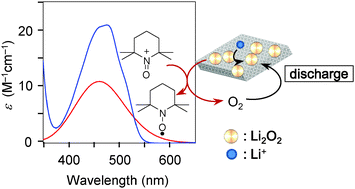
Precise knowledge of the discharge and charge reactions within Li–O2 batteries is an important aspect of developing highly efficient, rechargeable Li–O2 cells. We describe an analytical method capable of determining the quantity of Li2O2 in the cathode on the basis of the reaction of Li2O2 with an oxoammonium salt.

 Please wait while we load your content...
Please wait while we load your content...