Dipyreno- and diperyleno-anthracenes from glyoxylic Perkin reactions†
Abstract
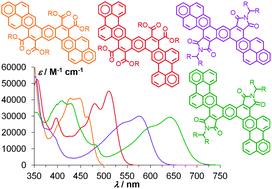
Twofold Perkin condensation of 2,5-dibromophenylene-1,4-diacetic acid with arylglyoxylic acids followed by cyclo-dehydrobromination leads to dipyreno- and diperyleno-anthracene tetraesters and diimides. The

 Please wait while we load your content...
Please wait while we load your content...