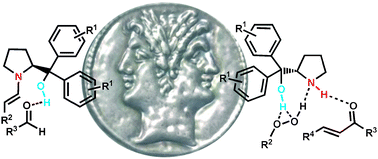
This feature article illustrates progress in asymmetric organocatalysis achieved by using readily available α,α-L-diaryl prolinols from 2009 up to middle 2012. This class of bifunctional organocatalysts has shown the ability to catalyze a variety of reactions such as epoxidation, cyclopropanation, α-sulfenylation of active methines, cross conjugate addition, direct aldol, cycloaddition and desymmetrization reactions. The most striking feature of these promoters is to enable either non-covalent or covalent activation of the reagents, thus distinguishing themselves as a rare example of organic promoters capable of encompassing alternative mechanistic pathways, namely enamine/iminium catalysis and Brønsted acid/base catalysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...