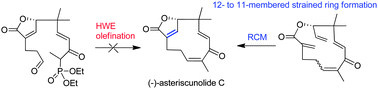
A 12-membered to a strained 11-membered ring: first stereoselective total synthesis of (−)-asteriscunolide C†
Abstract
The first stereoselective total synthesis of (−)-asteriscunolide C has been accomplished in 12 steps in a 16% overall yield. The key step is the conversion of a 12-membered to a strained 11-membered ring with one Z- and two E-double bonds employing a late stage

 Please wait while we load your content...
Please wait while we load your content...