Protein amyloids develop an intrinsic fluorescence signature during aggregation†
Abstract
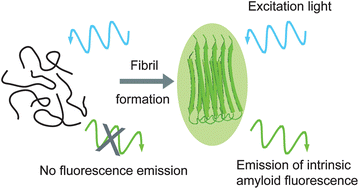
We report observations of an intrinsic fluorescence in the visible range, which develops during the aggregation of a range of polypeptides, including the disease-related human peptides amyloid-β(1–40) and (1–42), lysozyme and tau. Characteristic fluorescence properties such as the emission lifetime and spectra were determined experimentally. This intrinsic fluorescence is independent of the presence of aromatic side-chain residues within the polypeptide structure. Rather, it appears to result from electronic levels that become available when the polypeptide chain folds into a cross-β sheet scaffold similar to what has been reported to take place in crystals. We use these findings to quantify protein aggregation in vitro by fluorescence imaging in a label-free manner.
- This article is part of the themed collection: 2013's Top 25 most read Analyst articles

 Please wait while we load your content...
Please wait while we load your content...