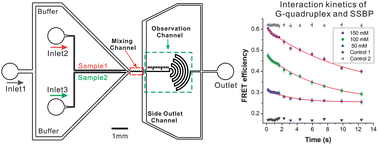
Kinetic measurement of biomacromolecular interaction plays a significant role in revealing the underlying mechanisms of cellular activities. Due to the small diffusion coefficient of biomacromolecules, it is difficult to resolve the rapid kinetic process with traditional analytical methods such as stopped-flow or laminar mixers. Here, we demonstrated a unique continuous-flow laminar mixer based on microfluidic dual-hydrodynamic focusing to characterize the kinetics of DNA–protein interactions. The time window of this mixer for kinetics observation could cover from sub-milliseconds to seconds, which made it possible to capture the folding process with a wide dynamic range. Moreover, the sample consumption was remarkably reduced to <0.55 μL min−1, over 1000-fold saving in comparison to those reported previously. We further interrogated the interaction kinetics of G-quadruplex and the single-stranded DNA binding protein, indicating that this novel micromixer would be a useful approach for analyzing the interaction kinetics of biomacromolecules.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...