Aqueous route for the synthesis of magnetite nanoparticles under atmospheric air: functionalization of surface with fluorescence marker†
Abstract
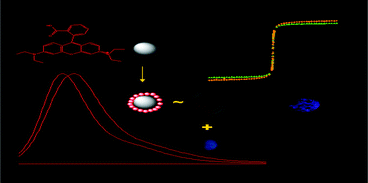
A simple chemical
* Corresponding authors
a
Department of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur-721302, India
E-mail:
ami@chem.iitkgp.ernet.in
A simple chemical

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
