Modulation of cross-linked actin networks by pH†
Abstract
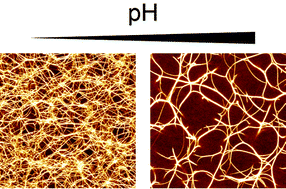
How cells orchestrate their complex cytoskeleton precisely in space and time is still far from understood. Recent studies suggest that local and transient variations of intracellular pH are critically involved in many important cytoskeletal processes. Yet, little is known about which

 Please wait while we load your content...
Please wait while we load your content...