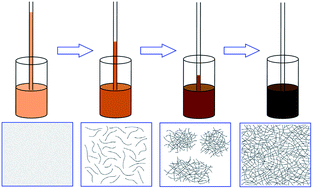
π-Conjugated organogels of poly(3-hexylthiophene) (P3HT) are prepared via the addition of a marginal solvent, anisole, into solutions of P3HT. We initiate a novel and facile route to determine the gelation threshold of P3HT nanowire dispersions by capillary measurements. The effects of the P3HT concentration (c), anisole volume fraction (φ) and temperature (T) on the phase behaviors are discussed. A thermodynamic c–φ phase diagram is constructed, in which the P3HT dispersion is divided into four regions: solution, sol, sol–gel blend and gel. The concentration and solvent dependent sol–gel transition kinetics shows that the gelation process could be accelerated via increasing c and φ. The gelation temperature gives an exponential relationship with the concentration of P3HT and the anisole volume fraction. Morphological studies reveal the formation process and the topological structure of the P3HT microgel clusters. Based on the above results, a three-stage physical scenario for the sol–gel transition of P3HT dispersions is proposed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...