Assembly and stability of α-helical membrane proteins
Abstract
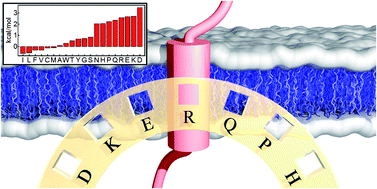
Grease to grease – this is how one might begin to describe the tendency of hydrophobic stretches in protein amino acid sequences to form transmembrane domains. While this simple rule contains a lot of truth, the mechanisms of membrane protein folding, the insertion of hydrophobic protein domains into the lipid bilayer, and the apparent existence of highly polar residues in some proteins in the hydrophobic membrane core are subjects of lively debate – an indication that many details remain unresolved. Here, we present a historical survey of recent insights from experiments and computational studies into the rules and mechanisms of α-helical membrane protein assembly and stability.

 Please wait while we load your content...
Please wait while we load your content...