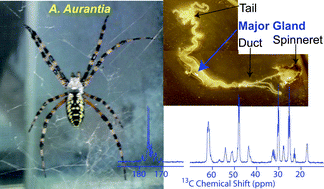
Spider silks display some of the most impressive mechanical properties of known fibrous materials. However, a molecular level understanding of how spiders store and convert the highly concentrated protein-rich fluid in the gland to an insoluble super fiber at the spinneret remains unanswered. In this report, we demonstrate the utility of High-Resolution Magic Angle Spinning (HR-MAS) NMR spectroscopy to study the molecular structure of the proteins within excised major ampullate (MA) glands from two orb weaving spiders, Nephila clavipes and Argiope aurantia. The spiders were fed various 13C-enriched amino acids to target different regions of the proteins and enable multi-dimensional, multinuclear HR-MAS NMR experiments. Comparisons are made between these HR-MAS results on MA glands and previous solid-state NMR data on spider dragline silk. The conformation-dependent 13C and 1H isotropic chemical shifts obtained in this study indicate that both dragline silk proteins, major ampullate spidroin 1 (MaSp1) and 2 (MaSp2), are present in a random coil conformation in the MA gland prior to fiber formation for both spider species.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...