Access to formally Ni(i) states in a heterobimetallic NiZn system†
Abstract
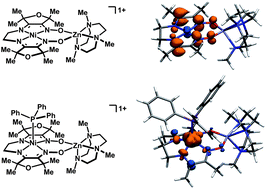
Heterobimetallic NiZn complexes featuring metal centers in distinct coordination environments have been synthesized using diimine–dioxime ligands as binucleating scaffolds. A tetramethylfuran-containing ligand derivative enables a stable one-electron-reduced S = 1/2 species to be accessed using Cp2Co as a chemical reductant. The resulting pseudo-square planar complex exhibits spectroscopic and crystallographic characteristics of a ligand-centered radical bound to a Ni(II) center. Upon coordination of a π-acidic ligand such as PPh3, however, a five-coordinate Ni(I) metalloradical is formed. The electronic structures of these reduced species provide insight into the subtle effects of ligand structure on the potential and reversibility of the NiII/I couple for complexes of redox-active tetraazamacrocycles.

 Please wait while we load your content...
Please wait while we load your content...