Terrylene bisimide-diarylethene photochromic switch†
Abstract
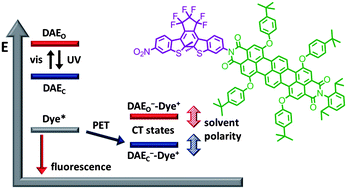
A new photochromic system 2o/2c based on a highly photostable terrylene bisimide (TBI) fluorophore and a diarylethene (DAE) photochrome has been synthesised and its photoswitching properties have been investigated by UV/vis absorption and

 Please wait while we load your content...
Please wait while we load your content...