Trimethyl lock: a trigger for molecular release in chemistry, biology, and pharmacology
Abstract
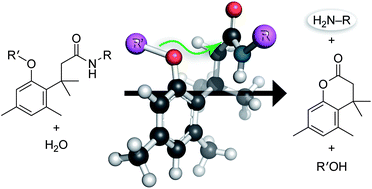
The trimethyl lock is an o-hydroxydihydrocinnamic acid derivative in which unfavorable steric interactions between three pendant methyl groups encourage lactonization to form a hydrocoumarin. This reaction is extremely rapid, even when the electrophile is an amide and the leaving group is an amino group of a small-molecule drug, fluorophore, peptide, or nucleic acid. O-Acylation of the phenolic hydroxyl group prevents reaction, providing a trigger for the reaction. Thus, the release of an amino group from an amide can be coupled to the hydrolysis of a designated ester (or to another chemical reaction that regenerates the hydroxyl group). Trimethyl lock conjugates are easy to synthesize, making the trimethyl lock a highly versatile module for chemical biology and related fields.
- This article is part of the themed collection: Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...