pH-induced mechanistic changeover from hydroxyl radicals to iron(iv) in the Fenton reaction†
Abstract
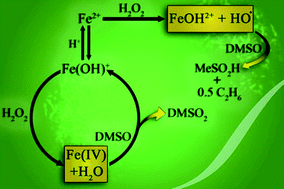
A major pathway in the reaction between Fe(II) and H2O2 at pH 6–7 in non-coordinating buffers exhibits inverse kinetic dependence on [H+] and leads to oxidation of dimethyl sulfoxide (DMSO) to dimethyl sulfone (DMSO2). This step regenerates Fe(II) and makes the oxidation of DMSO catalytic, a finding that strongly supports Fe(IV) as a Fenton intermediate at near-neutral pH. This Fe(IV) is a less efficient oxidant for DMSO at pH 6–7 than is (H2O)5FeO2+, generated by ozone oxidation of Fe(H2O)62+, in acidic solutions. Large concentrations of DMSO are needed to achieve significant turnover numbers at pH ≥ 6 owing to the rapid competing reaction between Fe(II) and Fe(IV) that leads to irreversible loss of the catalyst. At pH 6 and ≤0.02 mM Fe(II), the ratio of apparent rate constants for the reactions of Fe(IV) with DMSO and with Fe(II) is ∼104. The results at pH 6–7 stand in stark contrast with those reported previously in acidic solutions where the Fenton reaction generates hydroxyl radicals. Under those conditions, DMSO is oxidized stoichiometrically to methylsulfinic acid and ethane. This path still plays a role (1–10%) at pH 6–7.

 Please wait while we load your content...
Please wait while we load your content...