On the rational design of microwave-actuated organic reactions†
Abstract
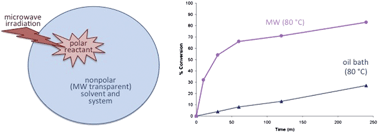
Microwave-actuated organic reactions are defined herein as chemical reaction systems for which microwave irradiation provides a clear benefit over conventional heating to the same temperature. This study is focused on a rationally designed, microwave-actuated reaction (thermal Friedel–Crafts

 Please wait while we load your content...
Please wait while we load your content...