Lewis acid enhancement by juxtaposition with an onium ion: the case of a mercury stibonium complex†
Abstract
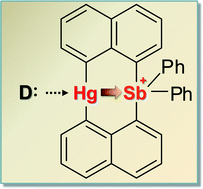
While diarylmercury derivatives (Ar2Hg) are usually not Lewis acidic, we have recently observed that bis(μ-1,8-naphthalenediyl)mercury(II)(bisphenylstibonium(V)) ([2]+), a compound that possesses a Ar2Hg moiety flanked on one of its sides by a stibonium unit, readily binds bromide or iodide ligands at the mercury center. To further investigate this behavior and understand its origin, we now report a series of results dealing with the coordination chemistry of [2]+. In particular, we show that this cation interacts with neutral donor ligands such as THF and DMAP to afford [2-THF]+ and [2-DMAP3]+, respectively, which have been isolated as [PF6]− salts. 1H and 199Hg NMR titration experiments carried out in DMSO-d6 indicate that the mercury center of [2]+ engages heavy halide anions to afford the corresponding complexes 2-Cl, 2-Br and 2-I whose stability constants are equal to 1890 (±10) M−1, 500 (±10) M−1, and 145 (±5) M−1, respectively. In the case of chloride, binding of a second halide ligand at antimony is observed leading to [2-Cl2]− which has been characterized as a [nBu4N]+ salt. Results obtained from titrating [2]+ against F− also indicate the formation of a complex, albeit with antimony as the primary anion binding site. Although the short Hg–Sb distances observed in these complexes (3.04–3.09 Å) remains essentially invariant, NBO calculations show a distinct strengthening of a 6s(Hg)→σ*(Sb–C) donor–acceptor interaction upon coordination of a halide to the mercury center. These NBO results also reveal weak 5d(Hg)→σ*(Sb–C) dative interactions which, as suggested by Hg L3 and Sb K-edge XANES measurements, are too weak to induce a measurable oxidation of the mercury center. In turn, we conclude that the enhanced Lewis acidity of the diarylmercury unit of [2]+ results from the presence of the stibonium moiety which provides a Coulombic pull for the coordination of Lewis bases while also drawing electron density away from the mercury atom via relatively weak orbital interactions.

 Please wait while we load your content...
Please wait while we load your content...