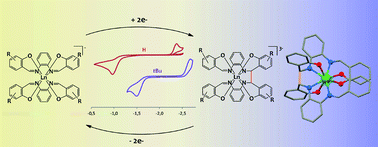
Multielectron redox chemistry, which is essential in metal catalysed chemical transformations, is not easily accessible in lanthanide complexes. Here we explored the reductive chemistry of lanthanide complexes with tetradentate Schiff bases acting as redox-active ligands with the objective of identifying new pathways to lanthanide multielectron redox transfer. The chemical reduction with alkali metals of heteroleptic [Nd(salophen)X] (salophen = N,N′-bis(salicylidene)phenylenediamine, X = I, OTf) and of a series of homoleptic K[Ln(Rsalophen)2] complexes of trivalent lanthanides has resulted respectively in the synthesis of the new dinuclear Nd(III) complex K2[Nd2(cyclo-salophen)(THF)2] and in the synthesis of a series of mononuclear lanthanide(III) complexes of general formula K3[Ln(bis-Rsalophen)] (R = H, Me, tBu). Ligandreduction and C–C bond formation are supported by X-ray crystal structures. Proton NMR studies demonstrate that the K2[Nd2(cyclo-salophen)(py)2] complex can transfer four electrons in the reaction with oxidizing agents such as AgOTf through the breaking of the two C–C bonds. Moreover the electrochemistry and reactivity of the mononuclear complexes K3[Ln(bis-Rsalophen)] show that they can act as formal two electron reductants and that their oxidation potential can be tuned by changing the substituents on the ligand. These results illustrate that Schiff bases provide a new way to introduce multielectron redox events at lanthanide centers and a new route to highly reactive mono- and polynuclear complexes of lanthanides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...