Concise total synthesis of (+)-gliocladins B and C†
Abstract
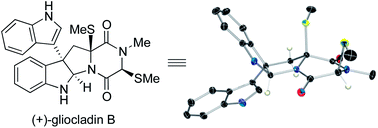
The first total synthesis of (+)-gliocladin B is described. Our concise and enantioselective synthesis takes advantage of a new regioselective Friedel–Crafts-based strategy to provide an efficient multigram-scale access to the C3-(3′-indolyl)hexahydropyrroloindole substructure, a molecular foundation present in a significant subset of epipolythiodiketopiperazine natural

 Please wait while we load your content...
Please wait while we load your content...