Catalytic C–H oxidation by a triazamacrocyclic ruthenium complex†
Abstract
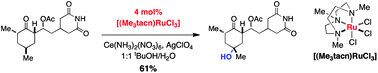
A method for oxygenation of tertiary and benzylic C–H bonds is described that uses 1–10 mol% (1,4,7-trimethyl-1,4,7-triazacyclo-nonane)ruthenium(III) trichloride as catalyst and ceric (IV) ammonium nitrate (CAN) as the terminal oxidant. The reaction is conveniently performed in aqueous solvent mixtures on substrates bearing a number of common, polar functional groups. The scope and efficiency of this process are comparable or superior to other known catalytic C–H oxidation technologies. Chemoselectivity trends and kinetic isotope effect data implicate a stepwise, radical-rebound mechanism for this transformation.

 Please wait while we load your content...
Please wait while we load your content...