A highly selective red-emitting FRET fluorescent molecular probe derived from BODIPY for the detection of cysteine and homocysteine: an experimental and theoretical study†
Abstract
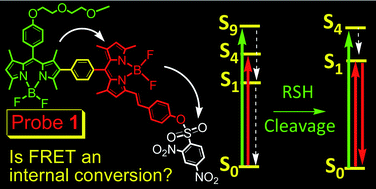
A red-emitting BODIPY-based fluorescent-resonance-energy-transfer (FRET) molecular probe 1 for selective detection of cysteine and homocysteine was designed. The fluorescence OFF–ON switch is triggered by cleavage of the 2,4-dinitrobenzensulfonyl (DNBS) unit from the fluorophore by thiols. The FRET energy donor (λabs = 498 nm, λem = 511 nm) is a parent BODIPY moiety and the energy acceptor is based on 4-hydroxylstyryl BODIPY moiety (λabs = 568 nm, λem = 586 nm). The unique C–C linker between the energy donor and acceptor was established using a Suzuki cross-coupling reaction. A polyether chain was also introduced into the probe to improve solubility in aqueous solution. While probe 1 itself is non-fluorescent, in the presence of cysteine or homocysteine a red emission at 590 nm is switched on (excitation at 505 nm), producing a pseudo-Stokes shift of up to 77 nm, which is in stark contrast to the small Stokes shift (ca. 10 nm) observed for typical BODIPY dyes. Excitation of the energy donor leads to the red emission from the acceptor of the probe, and demonstrates a high energy transfer efficiency. The probe was used for in vivo fluorescent imaging of cellular thiols. The fluorescence sensing mechanism of the probe and the photophysical properties of the fluorescent intermediates were fully rationalized by DFT calculations. The lack of fluorescence of probe 1 is attributed to the dark excited state S1 (oscillator strength f = 0.0007 for S0 → S1, based on the optimized S1 state geometry), which is due to the electron sink effect of the DNBS moiety. Cleavage of the DNBS moiety from the fluorophore by thiols re-establishes the emissive S1 state of the fluorophore (f = 1.4317 for S0 → S1), thus the red emission can be observed in the presence of thiols (fluorescence is turned on). The FRET effect of the probe was rationalized by DFT calculations which indicated that upon excitation into the S4 excited state (localized on the energy donor unit), the S1 state (localized on the energy acceptor, i.e. styryl-BODIPY) is populated via internal conversion (IC), thus red emission from the styryl-BODIPY energy acceptor is observed (Kasha's rule).

 Please wait while we load your content...
Please wait while we load your content...