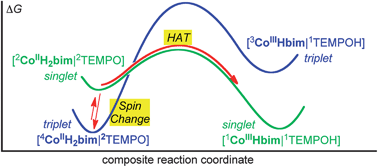
Described here are hydrogen atom transfer (HAT) reactions from high-spin cobalt(II) tris(2,2′-bi-2-imidazoline) (CoIIIIH22bim) to the hydrogen atom acceptors, 2,2,6,6-tetramethyl-1-piperidinyl-oxyl (TEMPO), 2,4,6-tri-tert-butylphenoxyl radical (tBu3ArO˙), and benzoquinone (BQ). The cobalt product is the oxidized and deprotonated, low-spin cobalt(III) complex (CoIIIIIIHbim), and the organic products are TEMPOH, tBu3ArOH, or hydroquinone, respectively. These reactions are formally spin forbidden because the spin state of the reactants is different from that of the products. For instance, quartet CoIIIIH22bim plus doublet RO˙ can have a triplet or quintet ground state, while the CoIIIIIIHbim + ROH product state is a singlet. Kinetics measured in the forward and reverse directions and thermochemical measurements provide a detailed picture of the reactions. The reactions are quite slow: the reaction of 10 mM CoIIIIH22bim with excess TEMPO requires roughly a day at ambient temperatures to reach equilibrium. This is 3400 times slower than the related reaction of the iron analogue FeIIIIH22bim, which is 2 kcal mol−1 more uphill. Mechanistic analyses show that the TEMPO reaction occurs by hydrogen atom transfer (HAT), and this is likely for the tBu3ArO˙ and BQ reactions as well. This is an unusually well defined spin-forbidden HAT system, which serves as a model for more complex multi-spin state HAT processes such as those suggested to occur in cytochrome P450 and metal-oxo model systems. In principle, HAT could occur before, after, or concerted with spin change. Computational studies indicate a reaction mechanism involving pre-equilibrium spin state interconversion of quartet 44CoIIIIH22bim to its doublet excited state 22CoIIIIH22bim, followed by spin-allowed HAT to the organic acceptor. This mechanism is consistent with the available kinetic, thermochemical and spectroscopic measurements. It indicates that the slow rates are due to the large change in geometry between CoIIIIH22bim and CoIIIIIIHbim, rather than any inherent difficulty in changing spin state. The implications of these results for other spin-forbidden or ‘two-state’ HAT processes are discussed.

 Please wait while we load your content...
Please wait while we load your content...