Mechanochemical processing of serpentine with ammonium salts under ambient conditions for CO2 mineralization†
Abstract
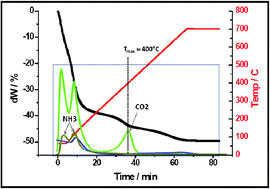
This paper assesses the suitability of mechanochemistry as a convenient low-energy processing option in CO2 mineralization. Whereas some success has been reported in milling alkaline earth-containing minerals under gaseous CO2, this work focuses instead on a purely solid-state approach towards two key objectives: (a) Mg extraction from serpentine using ammonium bisulfate; and (b) direct or indirect CO2 sequestration using ammonium bicarbonate in a natural extension of its role as “CO2 carrier” in the chilled ammonia scrubbing process. In Mg extraction work, dry milling of serpentine with ammonium bisulfate gave respectable yields (>60% Mg) as boussingaultite [(NH4)2Mg(SO4)2·6H2O] in 2 to 4 h. In CO2 sequestration, dry milling anhydrous magnesium sulfate with ammonium bicarbonate yielded only mixed sulfate products. Carbonation of the heptahydrate, epsomite, was found to proceed via ammonium magnesium carbonate hydrate [(NH4)2Mg(CO3)2·4H2O], which dissolves incongruently to yield nesquehonite [MgCO3·3H2O]. The modest conversion (∼30%) is probably due to equipartition of Mg into the double sulfate co-product. A similar route is followed in magnesia and brucite, in which the existence of an amorphous native carbonate precursor to nesquehonite in the same molar ratio (Mg : CO2 = 1) was inferred from inconsistency in the XRD intensities. This was largely responsible for the high carbonation yields in the unwashed products, ∼70% and ∼85% in MgO and Mg(OH)2, respectively, as confirmed by TG-FTIR. The same intermediate is probably formed in serpentine, but it is apparently soluble in the aqueous mineral environment. When the unwashed product is subjected to mild thermal consolidation, stable hydromagnesite [Mg5(CO3)4(OH)2·4H2O] is formed in ∼20% yield after milling for 16 h. Possible identities for the amorphous precursor are briefly considered.

 Please wait while we load your content...
Please wait while we load your content...