Hydrothermal synthesis of flower-like SnO2 particles consisting of single-crystalline nanorods through crystal growth in the presence of poly(acrylic acid)
Abstract
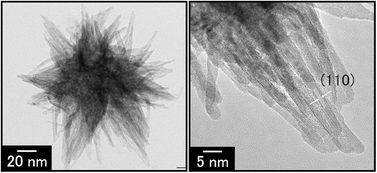
We prepared crystalline SnO2 particles from aqueous solutions of SnCl4 containing poly(acrylic acid) (PAA) by hydrothermal treatment at 150 °C. The morphology of the SnO2 particles depended on the concentration of PAA and the pH value of the precursor solutions. The flower-like SnO2 particles of ca. 100 nm in diameter consisting of needle-like units ca. 50 nm in size were obtained from the solution of [SnCl4·5H2O] = 0.1 M and [PAA] = 0.02 M containing the acidic

 Please wait while we load your content...
Please wait while we load your content...