Controlled Knoevenagel reactions of methyl groups of 1,3,5,7-tetramethyl BODIPY dyes for unique BODIPY dyes†
Abstract
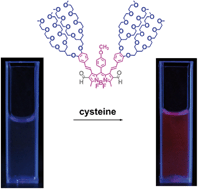
Formyl groups at 6- and 2,6-positions initiated Knoevenagel reactions of the methyl groups at the 7, and 1,7-positions of 1,3,5,7-tetramethyl
- This article is part of the themed collection: Organic Collection: from theory to synthesis, from molecules to materials, catalysis and beyond

 Please wait while we load your content...
Please wait while we load your content...