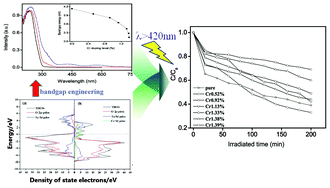
This work explores a solution route to the dual substitutions of a single dopant in a perovskite lattice, different from the sole substitution when using traditional solid state reactions. A series of NaTaO3 nanoparticles doped with Cr3+ were first synthesized by a hydrothermal method. The pure orthorhombic perovskite phase was retained regardless of the Cr3+ doping. At a lower doping level, <2.47 mol% Cr3+, Cr3+ primarily occupied the Ta5+ sites, creating certain oxygen vacancies. Strikingly, above this doping level, Cr3+ started to simultaneously substitute for both Na+ in 12-fold coordination sites and Ta5+ in 6-fold coordination states of the perovskite NaTaO3. This dual substitution is further indicated to give an increased surface area and a decreased bandgap energy. Even so, the higher dopant concentration resulted in a significant decrease in photocatalytic activity. These results were rationalized by theoretical simulation of the energy band structure using density functional theory, which unfolded that Na+ and Ta5+ doping by Cr3+ ions leads to the formation of new intermediate bands below the bottom of the conduction band, mainly due to the Cr 3d state, while the valence band was broadened due to the hybridization between the Cr 3d and O 2p states. Both factors made the absorption edge red-shift and increased the absorption coefficient in the visible region.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...