Redox processes in photochemistry of Pt(iv) hexahaloid complexes
Abstract
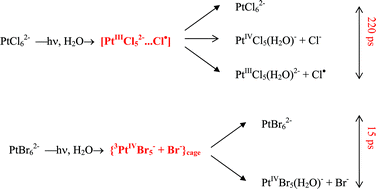
Ultrafast pump–probe spectroscopy (λpump = 405 nm) was applied to study the primary photochemical processes for PtCl62− and PtBr62− complexes in aqueous and alcohol solutions. For PtCl62−, an intermediate with a lifetime of ca. 200 ps was registered and identified as an Adamson radical pair [PtIIICl52−⋯Cl˙]. The transformations of the primary intermediate give rise to successive formation of different Pt(III) species. The reactions of Pt(III) results in chain photoaquation in aqueous solutions and reduction of Pt(IV) to Pt(II) in alcohol solutions. For PtBr62− complex, the previously reported (I. L. Zheldakov, M. N. Ryazantsev and A. N. Tarnovsky, J. Phys. Chem. Lett., 2011, 2, 1540; I. L. Zheldakov, PhD thesis, Bowling Green State University, 2010) formation of active 3PtBr5− intermediate is followed by very fast (15 ps) aquation of Pt(IV) in aqueous solutions and parallel reactions of solvation and reduction of Pt(IV) to Pt(II) in alcohol solutions. All the processes in alcohols are finished within 0.5 ns. The data of ultrafast experiments are supported by nanosecond laser flash photolysis and stationary photolysis.

 Please wait while we load your content...
Please wait while we load your content...