Direct experimental observation of the aggregation of α-amino acids into 100–200 nm clusters in aqueous solution†
Abstract
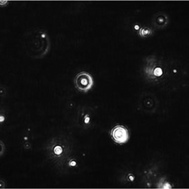
Spherical supramolecular aggregates of α-amino acids with a typical diameter of 100–200 nm are formed spontaneously after dissolution in water at a concentration of a few mM, i.e. well below the solubility limit. Their presence was shown by nanoparticle tracking analysis (NTA), atomic force microscopy (AFM), and ESI mass spectrometry (ESI-MS). There is a dynamic equilibrium between the aggregates and dissolved individual molecules which allows them to penetrate through dialysis membranes and filters. The same phenomenon was observed for para-amino salicylic acid and two dipeptides. Thermodynamic considerations suggest an entropy-controlled process.

 Please wait while we load your content...
Please wait while we load your content...