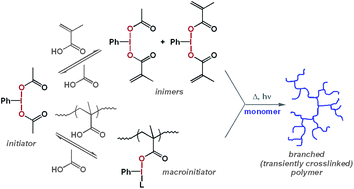
The acetoxy groups in (diacetoxyiodo)benzene, PhI(OAc)2, can exchange with methacrylic acid in various solvents yielding [(acetoxy methacryloyloxy)iodo]benzene or (dimethacryloyloxyiodo)benzene. The last two hypervalent iodine compounds can serve as inimers due to the presence of polymerizable moiety and the easy generation of radicals upon thermal or light-induced homolysis of the I–O bonds. PhI(OAc)2 was added to mixtures of methacrylic acid and methyl methacrylate, and upon heating to 80 °C, branched or transiently crosslinked polymers were formed. In homopolymerizations of methyl methacrylate initiated by PhI(OAc)2, i.e., in the absence of the monomer with carboxylic acid group, no branching or gelation was observed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...