Bis-semiquinone (bi-radical) formation by photoinduced proton coupled electron transfer in covalently linked catechol–quinone systems: Aviram's hemiquinones revisited†‡
Abstract
The structure and reactivity of a covalently linked
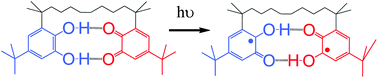
- This article is part of the themed collection: In honour of Professor Kurt Schaffner

 Please wait while we load your content...
Please wait while we load your content...