Aromatic aldols and 1,5-diketones as optimized fragrance photocages†
Abstract
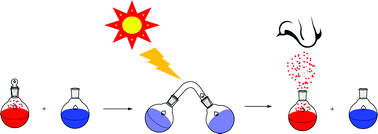
Aromatic aldols and 1,5-diketones with abstractable γ-hydrogen atoms are highly photoactive cage molecules for the release of
- This article is part of the themed collection: Photoremovable protecting groups: development and applications

 Please wait while we load your content...
Please wait while we load your content...