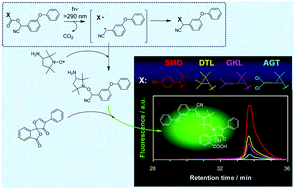
A novel radical trapping technique combined with a fluorescence spectroscopic analysis has been employed to investigate the radical intermediates produced by photodecarboxylation of four synthetic pyrethroids: fenvalerate (SMD), fenpropathrin (DTL), cyphenothrin (GKL), and cypermethrin (AGT). Under photoirradiation at >290 nm, all pyrethroids underwent direct photolysis via homolytic cleavage of the carbon–oxygen bonds in the ester groups. The consumed amount of a nitroxide free radical, as a trapping agent for the intermediate radical of a pyrethroid, was determined by ESR, which was the measure of the reaction yield of a photochemically generated α-cyano-3-phenoxybenzyl radical common to all pyrethroids. The reactivities of the pyrethroids studied was in the sequence of SMD ≫ DTL > GKL > AGT. Furthermore, nanosecond transient absorption spectroscopy demonstrated that geminate recombination of the radical pair within a solvent cage is the main deactivation route of the photochemically generated α-cyano-3-phenoxybenzyl radical common for all pyrethroids studied.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...